胃内滞留漂浮给药系统中使用草药根除幽门螺旋杆菌的研究进展
作者:Shreeraj H. SHAH, Jayvadan K. PATEL, Nirav V. PATEL
【关键词】 幽门螺旋杆菌; 胃滞留剂; 漂浮剂型; 草药; 黑柯子; 黄连素
Helicobacter pylori are very common patho?genic bacteria colonizing about half of all populations and associated with the development of serious gastroduodenal diseases like gastric lym?phoma, peptic ulcers and acute chronic gastritis. Current drug regimes are not wholly effective. Other problems related with the current drug regimes are lack of patient compliance, side effects and bacterial resistance. Thus, drug delivery to the site of residence in the gastric mucosa may help in solving the problems associated with the current drug therapy. Gastric retentive delivery systems potentially allow increased penetration and thus increased drug concentration at the site of action. Floating drug delivery systems, expandable or swellable drug delivery systems and bioadhesive systems are the major areas of interest to formulate gastroretentive drug delivery system against H. pylori. Generally, problems with these formulations are lack of specificity and the dependence on mucus turnover, so they fail to persist in the stomach. Gastric mucoadhesive systems are hailed as a promising technology to address this issue, penetrating the mucus layer and prolonging activity at the mucus?epithelial interface. Gastroretentive delivery strategies, specifically with regard to their application as a delivery system to target Helicobacter, are a very attractive field which can cure these troublesome infections.
H. pylori is a Gram?negative, microaerophilic, spiral and flagellated bacterium, with unipolar?sheathed flagella that provides motility. Its spiral shape and high motility allows it to penetrate mucus, resist gastric emptying and remain in the host gastrointestinal (GI) tract. It is now firmly established that infection with this bacterium is the cause of chronic active gastritis. Its isolation radically changed the conceptualisation of several chronic gastrointestinal illnesses including gastritis and peptic ulcers, and elimination of the causative organism became the goal of therapies [1]. Estimates from the WHO in 1994 claimed that about half of the world’s population was infected with H. pylori and although most infections are silent, a portion of the infected population will subsequently present with associated disease including chronic gastritis, peptic and duodenal ulcers. About 550 000 new cases a year of gastric cancerabout 55% of the worldwide totalwere attributed to H. pylori, and it was predicted that by 2020 to enter the top ten of leading causes of death worldwide[2, 3]. H. pylori is a very diverse specy and cancer risks may be increased with strains having virulence?associated genes (cytotoxin?associated gene, CagA), host genetics and environmental factors. The incidence of infection is higher in developing countries with up to 80%90% of adults being infected whereas in developed countries prevalence ranges from 10%50%[4].
1 Mechanism of H. pylori infections
Infection with H. pylori occurs predominately in childhood mainly between the ages 1 to 5, via oral ingestion of the bacterium, and lasts until the end of life with intrafamilial transmission being the major route in developed countries. The possible routes of transmission are food and water. The major feature of H. pylori infection is progressive injury to the gastric mucosa and its function[5, 6]. The bacterium adheres to the gastric epithelial cells, producing a direct injurious effect that is then amplified by production and release of a vacuolating cytotoxin (VacA)[7, 8]. H. pylori produces a variety of enzymes and is characterised by a high urease activity. Urea is broken down into bicarbonate and ammonia that protects the bacterium in the acid environment of the stomach. The ammonium ions produced can be toxic to the gastric superficial epithelial cells. Urease stimulates inflammatory cytokine production and activates mononuclear phagocytes. Although, after colonisation, the host immune defences are stimulated, and there is increased secretory IgA (sIgA) detected in the gastric mucosa and raised specific IgG, while the infected host is not able to eliminate the organism. Colonisation results in persistent gastric inflammation but the clinical course of infection can be very variable[9].
2 Current treatment of H. pylori infections
The treatment for eradication of H. pylori is complicated, requiring a minimum of two antibiotics in combination with gastric acid inhibitors. Although H. pylori is sensitive to many antibi?otics in vitro, no single agent is effective alone in vivo. Firstly, the bacterium resides below the gastric mucus adherent to the gastric epithelium and thus access of drugs to this site is limited. Sec?ondly, the strain may have acquired resistance to the commonly used antimicrobial drugs[10]. These infections are currently treated with a first?line triple therapy treatment, consisting of one proton pump inhibitor (PPI) and two antibiotics. None of the antibiotics used achieves sufficient eradication when used alone and also require adjuvant therapy[11]. This consists of agents increas?ing pH within the stomach to allow local action of antibiotics not active at low pH, and PPIs are used at a dose equivalent to 20 mg omeprazole twice daily. It was suggested that ranitidine bismuth citrate (RBC) regimens may be less influenced by antibiotic resistance than PPI?based therapies[12].
The most effective therapies combine two antibiotics including clarithromycin and amoxicillin with a gastric acid inhibitor. However, increasing resistance to current antibiotics is driving research to produce alternatives to the commonly used therapies. In addition to increasing levels of antibiotic resistance, the hostile environment of the stomach, reducing antibiotic bioavailability at the site of action, contributes to failures in treatment[13]. Current recommended regimes are not wholly effective, for example, triple therapy with bismuth, metronidazole and amoxicillin or tetracycline has an eradication efficiency of 60%80%, and patient compliance, side?effects and bacterial resistance can be problematic with this regime[14]. Alternatives proposed include quadruple therapies, based on, for example, colloidal bismuth subcitrate, tetracycline, metronidazole and omeprazole[15]. Patient compliance with such a complicated dosage regime could be improved by combining the therapies in a single dosage form, and a capsule containing bismuth biskalcitrate, metronidazole and tetracycline (Helicide) has been developed in an effort to improve patient compliance and has currently received approval[16]. There is concern regarding acquired resistance to two of the commonly prescribed antibiotics: clarithromycin and metronidazole. Although not as widespread, resistance to metronidazole can also be problematic but it can be overcome in some cases by lengthening the duration of treatment[17].
3 Drug delivery systems for gastric retention
Major problems in the eradication of H. pylori are the presence of antibiotic?resistant bacteria requiring multiple drugs with complicated dosing schedules and bacterial residence in an environment where high drug concentrations are difficult to achieve. In order to ensure that the therapy is adequately delivered to the unique niche of the gastric mucosa, development of oral dosage forms with prolonged gastric residence is desirable. Gastric retentive delivery systems have been studied for a number of years, and generally requirements of such strategies are that the vehicle maintains a controlled release of drug and exhibits prolonged residence time in the stomach. Overcoming the physiological barriers of the human GI tract is a major challenge facing successful development of gastric retentive systems and leads to problems with reproducibility. In addition to the thick protective mucus layer, gastrointestinal motility patterns are another obstacle facing drug delivery to the stomach. In the fasted state, the interdigestive myoelectric motor complex (IMMC) is a 2?hour cycle of peristaltic activity that regulates motility patterns[18]. Phase Ⅲ of the IMMC is also called the housekeeper wave and consists of strong, intense contractions designed to remove debris such as undigested food from the stomach[19, 20]. Gastric residence time will depend on which phase of IMMC is active. In the fed state, the stomach churns food to sizes less than 1 mm, which is then emptied to the duodenum. Type of the food determines its residence time in the stomach with liquids emptying rapidly and solids much more slowly. Gastric residence time is generally longer in the fed rather than fasted state. The gastric residence time of dosage forms is also influenced by posture, age, gender, disease status and concomitant medication. A number of different techniques have been explored to increase gastric retention including high density and magnetic systems, but the three main systems are floating systems, bio/mucoadhesive systems and swelling systems.
4 Floating drug delivery systems
Various approaches had been made since the late 1970s to utilise floating behaviour in order to prolong residence. Designs include hydrodynamically balanced systems (HBS), microspheres, gas?generating systems and raft?forming systems. Originally, such systems were proposed to reduce fluctuations in drug levels and provide sustained release as the duration of most oral sustained release preparations is 812 hours, due to a relatively short GI transit time[21, 22]. HBS has a bulk density lower than gastric fluids and contain one or more colloids formulated into a single unit with the drug and other additives, which swell on contact with water and facilitate floating[23, 24]. A density of less than 1.0 g/mL is required. A triple?layer floating tablet system was proposed containing a swellable gas?generating layer, a swellable drug?containing layer (with tetracycline and metronidazole) and a rapidly dissolving layer containing bismuth salts. The system was capable of providing sustained release of the antibiotics in vitro at pH 1.8 and demonstrated buoyancy in vitro, however no in vivo results are reported. Tablets containing a 1︰2 ratio of hydroxypropylcellulose to amoxicillin, with a gas?generating system, failed to improve efficacy. These large single?layer tablets remained buoyant in vitro but bioavailability was reduced to 80.5% as compared with conventional capsules in fasted humans[25]. Intersubject variability in gastric transit times with floating tablets and HBS results in unreliable and irreproducible residence times in the stomach and remains a significant problem with such systems. This can be addressed by using multiple?unit divided systems such as microspheres. As these can spread evenly through the stomach contents, they can avoid the problems of variable and early gastric emptying or bursting associated with the single?unit systems. Polymers used in formulation of floating multiple?units include caesin?gelatin acrylic polymers such as Eudragits and alginates[26]. Alginic acid is a polysaccharide consisting of D?mannuronic acid and L?guluronic acid. It forms a bioadhesive and stable gel with divalent cations such as calcium and the sodium salt been used in a variety of oral and topical formulations. Floating alginate systems such as Gaviscon form a buoyant gel which floats on the gastric contents alleviating symptoms of heartburn. Its stability in acidic media has made it a popular choice for gastric retentive delivery systems. For example, floating multiple units consisting of a calcium alginate core, separated from a calcium alginate/polyvinyl alcohol (PVA) membrane by an air compartment displayed prolonged gastric retention after a meal. Alginate beads are commonly prepared by extruding alginate, dropwise, into a solution containing Ca2+. The resultant beads are porous and can be used to encapsulate a variety of drugs with a wide range of physico?chemical properties[27]. Adequate control of drug release from such formulations often requires some modification to the matrix. For example, foam?based floating microspheres can be prepared by adding polypropylene foam powder to an organic solution containing dissolving polymer (Eudragit RS or polymethyl methacrylate, PMMA) and drug. Upon solvent evaporation, free?flowing microspheres are formed with extended release profiles[28]. Two types of alginate floating beads containing metronidazole were compared; one formulation contained chitosan and the other contained vegetable oils. In vitro release was complete from all formulations within two hours. Following administration to guinea pigs, it was concluded that after three hours chitosan?containing particles resulted in increased drug levels in the gastric mucosa as compared with metronidazole solution[29]. A multiple?unit floating dosage form formulated using calcium alginate was prepared by dropping sodium alginate solution into calcium chloride and the resultant particles were freeze?dried. Amoxicillin was incorporated into these beads by addition of drug to the calcium chloride solution. Once the sodium alginate was extruded into the solution, the resultant gel beads were left for thirty minutes before extraction and freeze?drying. Amylose was also added to the formulation in an attempt to reduce the release rate. Amoxicillin release showed an initial burst effect and the release was described by Higuchi kinetics, implying that it is controlled by diffusion of the drug through a porous matrix. Gamma?scintigraphic studies showed evidence of gastric retention of the floating beads in all seven subjects even following normal food intake.
A major limitation with such systems is the requirement for sufficient volumes of gastric acid within the stomach to enable the devices to float. It may be that using a single approach to localise delivery in the stomach may not be sufficient to resist the forces of gastric emptying. It was therefore envisaged that a floating dosage form with mucoadhesive polymers could extend the period of gastric retention, exploiting the retentive properties of the floating system and the ability of bioadhesive formulations to adhere to inflamed tissue. Floating, bioadhesive microsphere systems containing acetohydroxamic acid (AHA), a cytoplasmic urease inhibitor, were prepared. A solution of AHA and the acrylic polymer (Eudragit E) in ethanol/dichloromethane was added to an aqueous PVA solution to form an oil?in?water emulsion. The drug and polymer precipitated due to preferential diffusion of ethanol into the aqueous phase. After evaporation of the dichloromethane, the particles were dried and an air cavity was produced inside the spheres giving the particles the ability to float[30]. These particles were spray?coated with the mucoadhesive polymer, polycarbophil. Floating ability was demonstrated in vitro and demonstrated greater percentage growth inhibition of H. pylori in vitro than free drug. Release rates were extended due to the polycarbophil coating[31]. Similar preparations using polybisphenol?A carbonate as the coating polymer also showed buoyancy, extended drug release and inhibition of growth of H. pylori in vitro. Clearance of an inoculated strain of H. pylori from the stomach of gerbils following oral delivery of the encapsulated drug was shown to be better than free drug, presumably due to better retention. Although AHA has been shown to be effective at reducing gastritis in a Mongolian gerbil model, further studies are needed to prove that established antibiotics could also be successfully encapsulated into, and released from, such formulations and their efficacy demonstrated in human models[30].
5 Herbal and integrative drugs against H. pylori infections
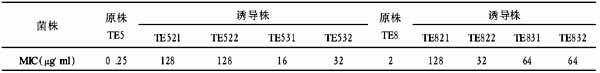
5.1 Black myrobalan The aqueous extract of black myrobalan (Terminalia chebula Retz) has been shown to have uniform antibacterial activity against ten clinical strains of H. pylori[32, 33]. This activity was bactericidal after 3 h and was stable after autoclaving. Although Sato and coworkers[34] reported gallic acid and ethyl gallate in T. chebula Retz and have shown antibacterial activity of ethanol extracts of this plant against both methicillin resistant and sensitive Staphylococcus aureus and other bacteria, the components of T. chebula Retz aqueous extracts responsible for the observed bacteriocidal activity remain unknown[35]. The antibacterial activity of aqueous extracts of black myrobalan against H. pylori was significantly higher than that of ether and alcoholic extracts. The aqueous extract preserved its antibacterial activity after autoclaving for 30 min at 121 ℃ and was inhibitory at 125150 mg/L. When the plant powder was tested directly against H. pylori, without grinding and (or) extraction and using Colombia Agar plates, the mean inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) values were 150 and 175 mg/L, respectively.
5.2 Ginger Ginger root (Zingiber officinale Rosc.) has been used traditionally for the treatment of gastrointestinal ailments such as motion sickness, dyspepsia and hyperemesis gravidarum, and is also reported to have chemopreventative activity in animal models[36]. The gingerols are a group of structurally related polyphenolic compounds isolated from ginger and known to be the active constituents. Since H. pylori is the pri?mary etiological agent associated with dyspepsia, peptic ulcer disease and the development of gastric and colon cancer, the anti?H. pylori effects of ginger and its constituents were tested in vitro[37]. A methanol extract of the dried powdered ginger rhizome, fractions of the extract and the isolated constituents, 6?, 8? and 10?gingerol and 6?shogoal, were tested against 19 strains of H. pylori, including 5 CagA?positive strains. The methanol extract of ginger rhizome inhibited the growth of all 19 strains in vitro with a MIC range of 6.25 to 50 μg/mL. One fraction of the crude extract, containing the gingerols, was active and inhibited the growth of all H. pylori strains with an MIC range of 0.78 to 12.5 μg/mL and with significant activity against the CagA?positive strains. These data demonstrate that ginger root extracts containing the gingerols inhibit the growth of H. pylori CagA?positive strains in vitro and this activity may contribute to its chemopreventative effects[38].
5.3 Turmeric Curcumin, a polyphenolic chemical constituent derived from turmeric (Curcuma longa L.), has been shown to prevent gastric and colon cancers in rodents[39]. Many mechanisms had been proposed for the chemopreventative effects, although the effect of curcumin on the growth of H. pylori has not been reported. H. pylori is a group 1 carcinogen and is associated with the development of gastric and colon cancer. A methanol extract of the dried powdered turme?ric rhizome and curcumin were tested against 19 strains of H. pylori, including 5 CagA?positive strains. Both the methanol extract and curcumin inhibited the growth of all strains of H. pylori in vitro with a minimum inhibitory concentration range of 6.2550 μg/mL. These data demonstrate that curcumin inhibits the growth of H. pylori CagA?positive strains in vitro, and this may be one of the mechanisms by which curcumin exerts its chemopreventative effects[40, 41].
5.4 Thyme A popular herbal remedy in ancient Egypt, Greece and Rome, thyme was mainly used for headaches, digestive problems, respiratory illness, and as a mood?enhancer. Researcher who investigated the antimicrobial properties of 21 essential oils against five important food?borne pathogens, including Escherichia coli (E. coli) noted that thyme was very effective at inhibiting the bacteria. Thyme extract was compared with several antibacterials; it had a significant inhibitory effect on H. pylori[42].
5.5 Licorice In a recent study at the Institute of Medical Microbiology and Virology, Ger?many, researchers found that licorice extract produced a potent effect against strains of H. pylori that are resistant against clarithromycin, one of the antibiotics typically used in the three antibi?otic treatment regimens[43]. The authors concluded that this study provides hope that licorice extract can form the basis for an alternative treatment for H. pylori infections[44].
5.6 Berberine Berberine is a plant alkaloid isolated from the roots and bark of several plants including golden seal, barberry, Coptis chinensis Franch. and Yerba mansa. Berberine?containing plants have been used medicinally in ayurvedic and Chinese medicine, and are known to have antimicrobial activity against a variety of organisms including bacteria, viruses, fungi, protozoans, helminths, and chlamydia. More recently, berberine had been demonstrated to be effective against H. pylori[45].
5.7 Goshuyn (Evodia rutaecarpaa Chinese herb) After testing no less than 113 Chinese herbs for anti?H. pylori activity in vitro, Japanese researchers identified goshuyn (Evodia rutaecarpa) as the most effective medical plant. Subsequently, they conducted a randomised clinical trial of two synthetic antibiotics versus the same combination plus goshuyn. The eradication rates were 60% and 80%, respectively[46].
5.8 Other Chinese herbals In an animal study or bacteriostatic test of 53 Chinese herbs, Zhang et al[47] found Coptis chinensis, Rheum palmatum, Panax notogenseng and Magnolia officinalis were effective against H. pylori. Prunus mume and Corydalis yanhusuo were moderate effective.
6 Conclusion
The gastric retention approaches as well as herbal drugs described here have applications for treatment of H. pylori infection although further development is required for each to be fully effective, especially in human studies. Overcoming the high mucus turnover rate and resulting limited retention times is a challenge for bioadhesive systems, and swelling systems must guarantee clearance from the stomach after a certain time to prevent any obstruction. The lack of availability of biocompatible chemical cross linking agents is a major stumbling block in the development of covalently cross?linked hydrogels. Floating systems are available commercially, and combination approaches, using floating behaviour and mucoadhesion, have also shown promise. Exploiting dual mechanisms of retention may provide the strength and reproducibility required to permit successful advancement in this field. So in future, a combination of herbal drugs with the novel drug delivery systems mentioned above, may lead to an important breakthrough in the herbal/integrative treatment of H. pylori infections.
7 Acknowledgement
I would like to acknowledge Dr. K. Pundarikakshudu for giving constant help to compile the information and in preparation of this article. I am also very much thankful to Prof. B.M. Peerzada and Prof. Manish Shah for constant encouragement.
【】
1 Andreica V, Sandica?Andreica B, Draghici A, Chiorean E, Georoceanu A, Rusu M. The prevalence of anti?Helicobacter pylori antibodies in the patients with ischemic heart disease. Rom J Intern Med. 2004; 42(1): 183?189.
2 Morgner A, Bayerdorffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa?associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am. 2000; 29(3): 593?607.
3 Kawahara Y, Mizuno M, Yoshino T, Yokota K, Oguma K, Okada H, Fujiki S, Shiratori Y. HLA?DQA1*0103?DQB1*0601 haplotype and Helicobacter pylori?positive gastric mucosa?associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2005; 3(9): 865?868.
4 Marshall B. Helicobacter pylori: 20 years on. Clin Med. 2002; 2(2): 147?152.
5 Suzuki H, Ishii H. Role of apoptosis in Helicobacter pylori?associated gastric mucosal injury. J Gastroenterol Hepatol. 2000; 15 Suppl: D46?D54.
6 Lehmann FS, Terracciano L, Carena I, Baeriswyl C, Drewe J, Tornillo L, De Libero G, Beglinger C. In situ correlation of cytokine secretion and apoptosis in Helicobacter pylori?associated gastritis. Am J Physiol Gastrointest Liver Physiol. 2002; 283(2): G481?G488.
7 Papini E, Zoratti M, Cover TL. In search of the Helicobacter pylori VacA mechanism of action. Toxicon. 2001; 39(11): 1757?1767.
8 Blanchard TG, Drakes ML, Czinn SJ. Helicobacter infection: pathogenesis. Curr Opin Gastroenterol. 2004; 20(1): 10?15.
9 Atherton JC. The pathogenesis of Helicobacter pylori?induced gastro?duodenal diseases. Annu Rev Pathol. 2006; 1: 63?96.
10 Iijima K, Sekine H, Koike T, Imatani A, Ohara S, Shimosegawa T. Long?term effect of Helicobacter pylori eradication on the reversibility of acid secretion in profound hypochlorhydria. Aliment Pharmacol Ther. 2004; 19(11): 1181?1188.
11 Chang FY, Lu CL, Chen CY, Luo JC, Jium KL, Lee SD. Effect of Helicobacter pylori eradicated therapy on water gastric emptying in patients with active duodenal ulcer. J Gastroenterol Hepatol. 2003; 18(11): 1250?1256.
12 Uygun A, Kadayifci A, Yesilova Z, Ates Y, Safali M, Ilgan S, Bagci S, Dagalp K. Poor efficacy of ranitidine bismuth citrate?based triple therapies for Helicobacter pylori eradication. Indian J Gastroenterol. 2007; 26(4): 174?177.
13 Batchelor H, Conway B,Williams RO. Targeting the infections within the gastrointestinal tract. In: Williams RO, Taft DR, McConville JT. Advanced drug formulation design to optimize therapeutic outcomes (Drugs and the pharmaceutical sciences). New York: Informa Healthcare. 2007: 217?244.
14 Lahaie R, Farley A, Dallaire C, Archambault A, Fallone CA, Ponich T, Hunt R, Oravec M, Whitsitt P, Van Zanten SV, Marcon N, Bailey R, Dumont A, Nguyen B, Desrochers S, Spénard J. Bismuth?based quadruple therapy with bismuth subcitrate, metronidazole, tetracycline and omeprazole in the eradication of Helicobacter pylori. Can J Gastroenterol. 2001; 15(9): 581?585.
15 De Boer WA. A novel therapeutic approach for Helicobacter pylori infection: the bismuth?based triple therapy monocapsule. Expert Opin Investig Drugs. 2001; 10(8): 1559?1566.
16 Kato M, Yamaoka Y, Kim JJ, Reddy R, Asaka M, Kashima K, Osato MS, El?Zaatari FA, Graham DY, Kwon DH. Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob Agents Chemother. 2000; 44(8): 2214?2216.
17 Streubel A, Siepmann J, Bodmeier R. Multiple unit gastroretentive drug delivery systems: a new preparation method for low density microparticles. J Microencapsul. 2003; 20(3): 329?347
18 Bernier JJ, Adrian V. Les aliments dans le tube digestif. Paris: Doin Editions. 1988: 11?24.
19 Dubernet C. Systèmes ? libération gastrique prolongée. In: Falson?Rieg F, Faivre V, Pirot F.Nouvelles formes médicamenteuses. Paris: Médicales Internationales, Lavoisier. 2004: 119?133.
20 Dressman JB, Lennern?s H. Oral drug absorption: prediction and assessment. New York: Marcel Dekker. 2000: 1?10.
21 Reddy LH, Murthy RS. Floating dosage systems in drug delivery. Crit Rev Ther Drug Carrier Syst. 2002; 19(6): 553?585.
22 Hwang SJ, Park H, Park K. Gastric retentive drug?delivery systems. Crit Rev Ther Drug Carrier Syst. 1998; 15(3): 243?284.
23 Erni W, Held K. The hydrodynamically balanced system: a novel principle of controlled drug release. Eur Neurol. 1987; 27(Suppl 1): 21?27.
24 Koller WC, Pahwa R. Treating motor fluctuations with controlled?release levodopa preparations. Neurology. 1994; 44(7 Suppl 6): S23?S28.
25 Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci. 1992; 81(2): 135?140.
26 Streubel A, Siepmann J, Bodmeier R. Floating microparticles based on low density foam powder. Int J Pharm. 2002; 241(2): 279?292.
27 Talukder R, Fassihi R. Gastroretentive delivery systems: hollow beads. Drug Dev Ind Pharm. 2004; 30(4): 405?412.
28 Murata Y, Sasaki N, Miyamoto E, Kawashima S. Use of floating alginate gel beads for stomach?specific drug delivery. Eur J Pharm Biopharm. 2000; 50(2): 221?226.
29 Whitehead L, Fell JT, Collett JH. Development of a gastroretentive dosage form. Eur J Pharm Sci. 1996; 4(Suppl 1): S182.
30 Umamaheshwari RB, Jain S, Bhadra D, Jain NK. Floating microspheres bearing acetohydroxamic acid for the treatment of Helicobacter pylori. J Pharm Pharmacol. 2003; 55(12): 1607?1613.
31 Umamaheswari RB, Jain S, Tripathi PK, Agrawal GP, Jain NK. Floating?bioadhesive microspheres containing acetohydroxamic acid for clearance of Helicobacter pylori. Drug Deliv. 2002; 9(4): 223?231.
32 Akhtar H, Virmani OP, Popli SP, Misra LN, Gupta MM, Srivastava GN, Abraham Z, Singh AK. Diction?ary of Indian medicinal plants. Lucknow: Central Institute of Medicinal and Aromatic Plants. 1992: 458.
33 Inamdar MC, Rajarama Rao MR. Studies on the pharmacology of Terminalia chebula. J Sci Ind Res. 1962; 21C: 345?348.
34 Sato Y, Oketani H, Singyouchi K, Ohtesuro T, Kihara M, Shibata H, Higuti T. Extraction and purification of effective antimicrobial constituents of Terminalia chebula RETS. against methicillin?resistance Staphylococcus aureus. Biol Pharm Bull. 1997; 20(4): 401?404.
35 Malekzadeh F, Ehsanifar H, Shahamat M, Levin M, Colwell RR. Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int J Antimicrob Agents. 2001; 18(1): 85?88.
36 Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007; 247(2): 167?181.
37 Presser A. Pharmacists’s guide to medicinal herbs. Petaluma, CA: Smart Publications. 2000: 147?154.
38 Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of CagA+ strains of Helicobacter pylori. Anticancer Res. 2003; 23(5A): 3699?3702.
39 Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002; 22(6C): 4179?4181.
40 Nostro A, Cellini L, Di Bartolomeo S, Di Campli E, Grande R, Cannatelli MA, Marzio L, Alonzo V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother Res. 2005; 19(3): 198?202.
41 Foryst?Ludwig A, Neumann M, Schneider?Brachert W, Naumann M. Curcumin blocks NF?kappaB and the motogenic response in Helicobacter pylori?infected epithe?lial cells. Biochem Biophys Res Commun. 2004; 316(4): 1065?1072.
42 Smith?Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food?borne pathogens. Lett Appl Microbiol. 1998; 26(2): 118?122.
43 Krausse R, Bielenberg J, Blaschek W, Ullmann U. In vitro anti?Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. J Antimicrob Chemother. 2004; 54(1): 243?246.
44 Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Anti?Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002; 71(12): 1449?1463.
45 Mahady GB, Pendland SL, Stoia A, Chadwick LR. In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother Res. 2003; 17(3): 217?221.
46 Higuchi K, Arakawa T, Ando K, Fujiwara Y, Uchida T, Kuroki T. Eradication of Helicobacter pylori with a Chinese herbal medicine without emergence of resistant colonies. Am J Gastroenterol. 1999; 94(5): 1419?1420.
47 Zhang L, Yang LW, Yang LJ, Zheng XG, Pan CL. Relation between Helicobacter pylori and pathogenesis of chronic atrophic gastritis and the research of its prevention and treatment. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992; 12(9): 521?523, 515?516. Chinese with abstract in English.